— Luca Panariello, UCL
Ultrasound is sound waves with frequencies higher than the upper audible limit of human hearing. It is no different from “normal” audible sound in its physical properties, except that humans cannot hear it. The feasibility of converting sound into chemistry was demonstrated more than 80 years ago, when Lord Rayleigh postulated the existence of cavitation bubbles. Ultrasound has frequencies ranging from 20 kHz to 1 GHz and sound velocities in liquids of ∼1500 m/s. This results in acoustic wavelengths ranging from 10 cm to 10-4 cm which is far above molecular and atomic dimensions. Consequently, ultrasound does not directly interact with chemical compounds on a molecular level. Sonochemistry derives from another way of concentrating ultrasonic energy: acoustic cavitation.
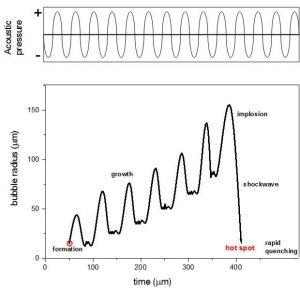
Figure 1 – Bubble evolution during ultrasonic irradiation
Acoustic cavitation appears in liquids at high and moderate intensities of ultrasonic irradiation. A liquid expands during the expansion (negative) phase of an ultrasonic wave. If the negative pressure induced by the wave in the liquid is high enough such that the average distance between the molecules exceeds the critical molecular distance necessary to hold the liquid intact, the liquid breaks down and creates voids or cavities; these are cavitation bubbles. Once produced, these bubbles may grow until the maximum of the negative pressure has been reached (Figure 1). In the succeeding compression cycle of the wave however, they will be forced to contract and some of them may even disappear totally: collapsing. The collapse of the bubbles occurring during cavitation is more rapid than thermal transport and generates localized hot spots, with temperature in the order of 5000 K, pressures of 1000 atm and heating rates of more than 1010 K/s 1. These severe conditions allow the activation of reaction mechanisms otherwise inexplicable.
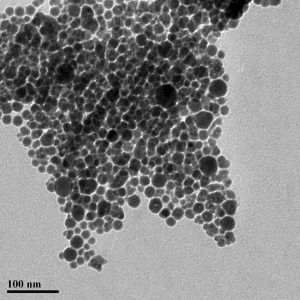
Figure 2 – Sonochemically synthetised Iron nanoparticles
Sonochemistry has been proven to potentially be a useful tool towards green synthesis of many different nanomaterials 2,3. An example is the sonochemical synthesis of iron colloids4, that otherwise would require severe conditions5. Through ultrasonic irradiation it is possible to achieve these synthesis at room temperature.
In the scope of process intensification, the challenge we are now facing is the development of continuous reactors based on these concepts.
- Flint, E. B. & Suslick, K. S. The temperature of cavitation. Science 253, 1397–1399 (1991).
- Shchukin, D. G., Radziuk, D. & Möhwald, H. Ultrasonic Fabrication of Metallic Nanomaterials and Nanoalloys. Annu. Rev. Mater. Res. 40, 345–362 (2010).
- Hinman, J. J. & Suslick, K. S. Nanostructured Materials Synthesis Using Ultrasound. Top. Curr. Chem. 375, 12 (2017).
- Suslick, K. S., Fang, M. & Hyeon, T. Sonochemical synthesis of iron colloids. J. Am. Chem. Soc. 118, 11960–11961 (1996).
- Huber, D. L. Synthesis, properties, and applications of iron nanoparticles. Small 1, 482–501 (2005).




