Collapsing of the rapid and nearly adiabatic small bubble driven by a standing ultrasound heats inside the bubble up and produces enormous energy. This energy is able to initiate the chemical reactions (sonochemistry) and to emit light (sonoluminescence). Spectroscopic measurements reveal that collapsing of a bubble leads to high temperatures (>5000 K) and high pressures (>1000 bar) [1]. Sonoluminescence was first observed from water by Frenzel & Schultes (1934). As with sonochemistry, sonoluminescence originates from the acoustic cavitation. Sonoluminescence is divided into two classes: multiple-bubble and single-bubble sonoluminescence (MBSL and SBSL, respectively).
Liquids contain large number of particulates inside that behaves as a nuclei in order to initiate cavitation. Then the cavitation field is formed by standing or propagating acoustic wave. This field contains large number of bubbles interacting each other and distributed all over the liquid. If the cavitation is sufficiently intense to generate sonoluminescence, this phenomenon is called as multiple-bubble sonoluminescence [2]. Actual acoustic pressure effecting the each bubble is different due to the spatial distribution of applied acoustic pressure; therefore, the number of photons released collapsing of per bubbles are not equal to each other. In Figure 1, horn type ultrasonic transducer (located at the top) is used to cavitate Xe gas filled H2SO4 solution.
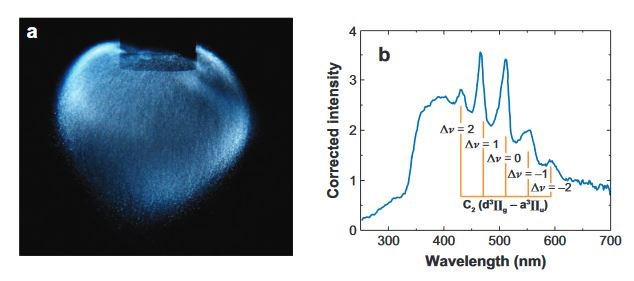
Figure 1: (a) True-color photograph (no external lighting) of a cloud of sonoluminescing bubbles generated with an ultrasonic horn in 96 wt% H2SO4 saturated with Xe gas. The diameter of the Ti horn tip is 1 cm. Photograph courtesy of N.C. Eddingsaas. (b) Multibubble sonoluminescence spectrum generated from the sonication of Ar saturated dodecane at 4 oC. The emission is assigned to the Swan band of C2 and the transitions responsible for each line are shown.
The acoustic force on a single bubble equates against its buoyancy under the suitable conditions, stabilizing (holding) the bubble (stable) in the liquid by acoustic levitation. Radius of such a bubble is reasonably small when the acoustic wavelength is considered. For example, at acoustic wavelength of 20 kHz, the maximum radius of corresponding bubble is about 50 µm [3]. Single stable gas filled bubble driven by large amplitude pulsation produces sonoluminescence during every duty cycle. This emission of light from an acoustically trapped single bubble is called as single-bubble sonoluminescence. Sonoluminescence from gas filled single bubble assists to discover “the secret life of bubbles”, dynamic characteristics of the bubbles both in theoretical and experimental aspects more detail.
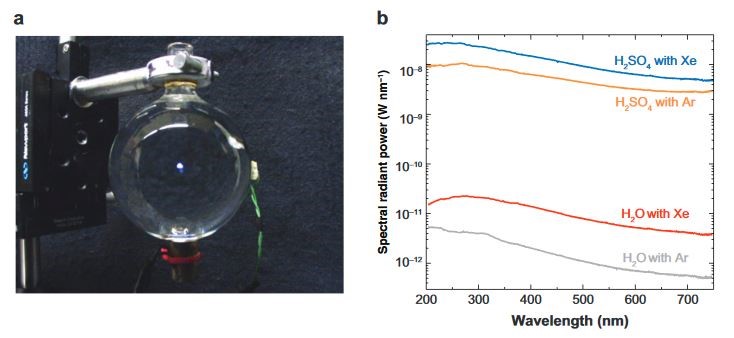
Figure 2: (a) Photograph of single-bubble sonoluminescence (SBSL) (center white spot), generated in 85 wt% H2SO4 partially regassed with Xe. The diameter of the spherical quartz cell is 6 cm. The exposure time was 2 s, and the photograph was taken in a fully lit room. (b) SBSL spectra from degassed water partially regassed with Ar or Xe compared to 85 wt% H2SO4. The applied acoustic pressure was optimized to give the maximum spectral radiant power for each [4].
Ekim Sarac, University of Göttingen
References
- Tandiono, Ohl, S-W., Ow, D. S. W, Klaseboer, E., Wong, V. V., Dumke, R., and Ohl, C-D. (2011). “Sonochemistry and Sonoluminescence in Microfluidics”. PNAS, vol 108, pp 5996-5998.
- Suslick, K. S. and Flannigan, D. J. (2008). “Inside a Collapsing Bubble: Sonoluminescence and the Conditions During Cavitation”. Annu. Rev. Phys. Chem., vol 59, pp 659-83.
- Suslick, K. S., Didenko, Y., Fang, M. M., Hyeon, T., Kolbeck, K. J., McNamara III, W. B., Mdleleni, M. M. And Wong, M. (1999). “Acoustic Cavitation and Its Chemical Consequences”. Phil. Trans. R. Soc. Lond. A, vol 357, pp 335-353.
- Flannigan, D. J., and Suslick, K. S., (2005). “Plasma formation and temperature measurement during single-bubble cavitation”. Nature, vol 434, pp 52-55.




